-
 recovering gold from gold-containing waste liquids
recovering gold from gold-containing waste liquidsMethods for Recovering Gold from Gold-Containing Waste Liquids:
Gold-containing waste liquids include electroplating waste liquids, which mainly consist of cyanide plating waste solutions and sulfuric acid gold plating solutions, as well as nitric acid waste solutions, chloride waste solutions, and various gold-containing wash waters. Electroplating waste solutions typically contain higher levels of gold, with acidic gold plating waste solutions commonly having 4~12 g/L of gold and alkaline gold plating waste solutions reaching up to 20 g/L.
-
 etching solution leaching
etching solution leachingEtching Solution Leaching, Electrolytic Copper Regeneration System:
The copper leaching process generates a large amount of leaching waste, which contains copper ions (100-170 g/L), ammonia, and sodium chloride. Traditional handling methods involve selling the waste as is by PCB manufacturers, who use it as raw material to produce foam copper and other products via precipitation. However, this method fails to effectively utilize the excess ammonium ions and small amounts of copper ions, leading to resource wastage.
Our Innovative Process:We have adopted a solvent leaching - reverse leaching - electroplating process for the regeneration of leaching waste. This approach uses leaching to separate copper ions from the leaching waste without causing damage, achieving a clean separation. The leach solution is then separated into its components through fractionation. Once the leaching performance is restored, the entire solution is returned to the copper production line for reuse. Finally, we utilize electroplating technology to process the reverse-leached electrolyte, producing copper plates with over 99.5% purity.
-
 colloid palladium adsorption recovery method
colloid palladium adsorption recovery methodColloid Palladium Adsorption Recovery Method:
Acidic colloid palladium is generated in the early activation treatment solution within the hole of the circuit board. Its composition consists of a sodium chloride bath and weak acid stabilization, as it has Sn encapsulation.
Reasons for Encapsulation with Tin:Due to the tin encapsulation, it's necessary to break through this layer to enable adsorption of palladium. Conventional resins or activated carbons are ineffective in this process, as they cannot easily penetrate or effectively adsorb palladium.
Our Custom-Built Activated Carbon:
To address this challenge, we have specifically developed and produced activated carbon optimized for the adsorption of acidic colloid palladium. This activated carbon achieves efficient recovery of colloid palladium, with an adsorption capacity ranging from 5 to 20 grams per liter.
-
 99.99% pure silver metal
99.99% pure silver metalHigh-Efficiency, Environmental-Friendly Silver Refining Equipment Uses Advanced Electrorefining Technology
The silver refining equipment employs advanced electrorefining technology. Using 925 silver as the anode and titanium as the cathode, the process operates in electrolytic solution to reduce silver ions at the cathode into 99.99% pure silver metal. This method surpasses traditional nitric acid refining methods in several key aspects:
Reduction of Nitric Acid Use and Discharge: The equipment significantly reduces reliance on and environmental impact from nitric acid, minimizing pollution risks.
Importance of Environmental Protection: For modern industries, where environmental protection is a paramount consideration, this reduction in nitric acid use offers substantial value.
Customization and Flexibility: The device can be tailored to meet production capacity requirements, offering high adaptability. This flexibility not only caters to differing scales of enterprises but also enhances operational efficiency and lowers production costs.
Simplicity and Ease of Use: The equipment is designed for ease of operation, combining the advantages of simplicity and effectiveness in silver refining.
In summary, this equipment excels in terms of environmental protection, customization capabilities, and user-friendliness, making it a highly efficient and sustainable solution for silver refining. Its adoption brings significant commercial value to relevant industries by enhancing resource utilization and operational efficiency while minimizing environmental impact.
-
 extraction of gold from micro gold containing waste liquid
extraction of gold from micro gold containing waste liquidA complete system for resin adsorption, analysis and extraction of gold from micro gold containing waste liquid:
Using specialized gold ion exchange resin, the three-stage adsorption process ensures that the final effluent concentration of gold is below 0.02 ppm, achieving a recovery rate of over 99%.
After the resin becomes saturated, the elution system is activated to desorb the absorbed gold ions from the resin.
The eluted solution undergoes thorough washing of the resin before being recirculated back into the electrolytic tank for electrorefining.
The eluate is then returned to the storage tank and ready for reuse in subsequent operations.
-
 Ion palladium solution chemical reduction method
Ion palladium solution chemical reduction methodIon palladium solution chemical reduction method: Production line wastewater containing ion-balanced palladium acid is collected through dedicated pipes and introduced into the original water storage tank. Acid and alkali wastes should be separated. After pumping with a water pump, the liquid is adjusted to a certain pH value in the regulation pool. Then, it is drawn into the reaction pool, where a specific palladium fluoride reagent is added slowly to precipitate beryllium out of the solution. The palladium powder settles, undergoes filtration and calcination, producing high-purity oxides of palladium. After reduction, the filtrate contains less than 1 ppm of palladium. Overflow solution from washing is treated with imported specialized palladium ion resin adsorbent material. Through a three-stage adsorption column, the recovery rate can reach 95% or higher. Adsorbed beryllium can be either leached using elution or electrorefined after desorption or burned to recover the absorbed palladium.
-
 etching solution extraction and copper electrowinning
etching solution extraction and copper electrowinningetching solution extraction and copper electrowinning:
The printed circuit board (PCB) corrosion process generates a large amount of corrosion sludge. This sludge contains copper ions ("100-170g/L" ammonia, salts, and ammonium chloride). The traditional handling method is that PCB manufacturers sell the sludge as waste液 and use it as raw material to produce sponge copper and other products via precipitation methods. However, a large amount of ammonia ions and a small amount of copper ions cannot be effectively utilized, leading to resource waste.
First process: This process uses direct electrowinning. Direct electrowinning is a method where the corrosion sludge is directly Electrolytic deposition extraction high-purity electrolytic copper, reducing the copper ion concentration in alkaline corrosion sludge and then performing component optimization before reuse in production lines. If recycling utilization is not required, copper can be removed on-site for disposal, and comprehensive utilization can reduce treatment costs. Pollutants are treated to meet required concentrations.
Second process: This process uses solvent extraction - reverse extraction - electrorefining for the regeneration of corrosion sludge. The copper ions in the corrosion sludge are concentrated via leaching using a solvent extraction method, achieving non-destructive separation. The leach liquid and remaining are then mixed and optimized to restore the corrosion performance before being returned to the production line for use. Finally, electrorefining is performed on the reverse-extracted electrolyte to obtain copper plates with purity of 99.5% or higher.
-
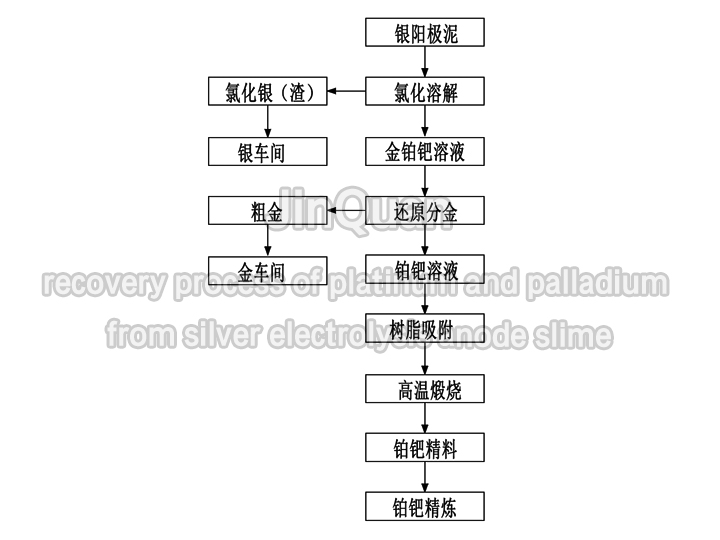 recovery process of platinum and palladium
recovery process of platinum and palladiumrecovery process of platinum and palladium from silver electrolysis anode slime
The majority of platinum and part of the tantalum in the impure silver anode remains in the anodic slime. generally, platinum and palladium in the anodic slime are recovered.
-
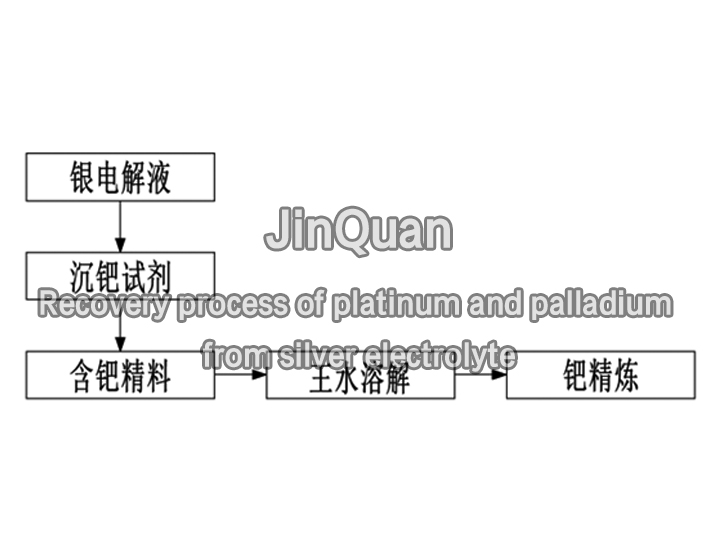 recovery process of platinum and palladium
recovery process of platinum and palladiumRecovery process of platinum and palladium from silver electrolyte
When silver is electrolyzed, some tantalum from the impure silver anode will enter the electrolyte. Due to the similar electrode potentials of tantalum and silver, when the concentration of tantalum in the electrolyte exceeds 20 ppm, it will be deposited on the cathode.







