-
 Method for Extracting Gold from Copper Oxidized Ore
Method for Extracting Gold from Copper Oxidized OreMethod for Extracting Gold from Copper Oxidized Ore:
This method, belonging to wet metallurgical technology, involves breaking down and classifying copper oxidized ore into fragments.It then undergoes alkaline treatment, followed by the addition of certain proportions of thiourea and cyanide. This process achieves inhibition of copper dissolution while promoting selective gold dissolution.
Finally, the elution solution uses conventional activated carbon to adsorb and extract gold.
This method has high gold recovery rates, low reagent consumption, and a low cost, making it easy to industrialize with good economic benefits.
-
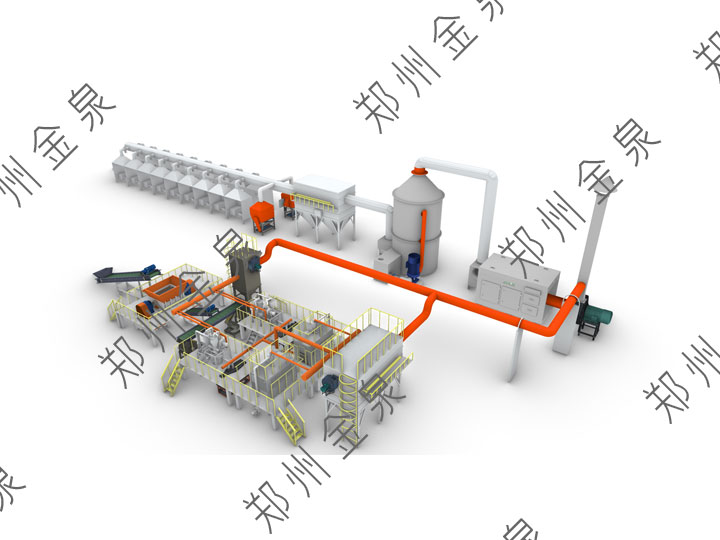 Regenerated Copper in a Single-Step Process
Regenerated Copper in a Single-Step ProcessThe Comprehensive Utilization of Regenerated Copper in a Single-Step Process:
In this single-step process, regenerated copper is directly used in the smelting furnace to melt into copper solution—after oxidation-reduction refining melting, obtain high-performance copper solution—cast into copper electrolytic sheets;
Electrorefining Process: Electrorefining of copper cathodes—1st stage cathode copper and precious metal copper slag;
Recycling of Copper Slag: Copper slag is oxidized and roasted—divided into copper (copper solution goes to the copper electrorefining room for recycling)—divided into gold (reduced to raw gold)—divided into silver (silver is reduced to raw silver)
The energy consumption of regenerated copper is approximately 0.8-1.2 kilograms of standard coal per ton of copper, while that of native copper is about 2.5-3 kilograms of standard coal per ton of copper; the CO₂ emission of regenerated copper is only 30%-40% that of native copper, with significant advantages.
-
 Extracting Gold from Difficult-to-Leach Ores
Extracting Gold from Difficult-to-Leach OresThe Method of Simultaneous Leaching with Chloride-Oxidized Complex for Extracting Gold from Difficult-to-Leach Ores: This method falls under wet metallurgical techniques.
First, the ore is shattered into particles and mixed with sodium hydroxide (NaOH), sodium hypochlorite (NaClO), and water to form a slurry. Air is then introduced into the slurry under stirring conditions to enhance leaching efficiency through ultrasonic assistance.
During the enhanced leaching process, hydrogen peroxide (H2O2) is periodically added. The cooperative effect between NaOH and hydrogen peroxide significantly enhances the oxidation-reduction reaction, enabling faster gold extraction while minimizing reagent costs. This method achieves a gold leaching rate of over 98%, eliminates potential environmental impacts from sodium hypochlorite, and shortens the overall leaching process in a single step.
-
 The Leaching-Precipitation-ElectroREFINING Process
The Leaching-Precipitation-ElectroREFINING ProcessTreatment of Copper Oxide Mines Using the Wet Metallurgical Leaching-Precipitation-ElectroREFINING Process:
A wet metallurgical process is employed for the recovery of copper from copper oxide mines using a leaching-precipitation-electroREFINING method. This approach is particularly well-suited for copper deposits where mining points are numerous, ore quantities are limited, and the copper oxides are disseminated in nature.
One key feature of this method is its ability to perform in-place leaching with sulfuric acid following heap leaching. Afterward, neutralization of the leach solution with a base precipitates copper as Cu(OH)2, which is then washed and filtered. The copper-bearing material from the filter is redissolved in sulfuric acid to form a copper sulfate solution.
The standard leaching-precipitation-copper process is then applied, followed by a high-purity copper recovery step, typically involving advanced electroREFINING methods to achieve a purity level exceeding 99.9%.
This approach offers several advantages:
It eliminates the need to transport mineralized material and directly processes the ore on-site, significantly reducing transportation costs.
The process is cost-effective and highly efficient compared to conventional mining practices.
-
 Raw Silver Electrolytic Refining
Raw Silver Electrolytic RefiningRaw Silver Electrolytic Refining:
Using raw silver as the anode and nitric acid silver solution, high current density electrolysis is employed to produce silver powder with a purity of over 99.99%.
The anode residue from electrolysis undergoes washing before being placed directly into the anodic electrolytic cell to produce silver powder of over 99.99% purity.
The spent electrolyte undergoes silver oxide filtration, achieving quality standards for reusable electrolyte, which is then reintroduced to the electrolysis process.
-
 Customized recovery of high-value platinum group
Customized recovery of high-value platinum groupProcess tailored for different carriers in petrochemical and pharmaceutical industries:
A four-step process—incineration, refining, dissolution, and precipitation—is employed.
This method achieves the highest platinum group recovery rate of 99%, converting the carrier into building material ultrafine powder to efficiently recycle and reuse resources.
-
 The Chemical Refining Method For Gold
The Chemical Refining Method For GoldThe chemical refining method :
The chemical refining method for gold generally uses aqua regia or sodium chlorate and hydrochloric acid to dissolve crude gold, and then adds reducing agents such as sodium sulfite to control the potential reduction, obtaining 99.99% gold.
-
 Advantages of JinQuan Gold Electrolysis
Advantages of JinQuan Gold ElectrolysisAdvantages of JinQuan Gold Electrolysis:
High Stability in Product Quality: The product can achieve a gold ingot purity of 99.99%, and even higher purity (up to 99.999%) can be produced based on specific requirements.
Low Production Costs: Consumes 150 liters of HCl per ton of gold, ensuring cost-effectiveness.
High Current Density Electrolysis Technology: Utilizes a current density range of 1200-1500 A/m², achieving high production efficiency with an electrolysis cycle duration of 10-22 hours (depending on the thickness of the copper cathode).
Minimized Gold Usage in Electrolyte: The electrolyte uses about 96 liters per day, containing 120-150 grams of gold per liter. This results in minimal gold consumption, with up to 14.4 kilograms of gold used per ton.
Transparent Hood Design: Provides a clean and safe work environment, enhancing both functionality and aesthetics. The entire system occupies an area of approximately 2 square meters.
High-Performance Materials: The main components are made of high-performance polypropylene, while the electrodes (both anode and cathode) feature a titanium-copper composite, ensuring excellent electrical conductivity.
PLC Automated Control System: Incorporates a PLC-based automation system with touch-screen control for managing power supply, current, voltage settings, heating, and cycling functions. The system includes monitoring capabilities for copper dissolution temperature, electrolytic voltage, and current. It can automatically shut down the power supply in case of parameter anomalies and stores electrolysis parameters for real-time query and analysis. The system operates with high safety and convenience.
This comprehensive design ensures efficient and reliable gold-silver electrolysis processes, meeting both technical and operational requirements.
-
 Advantages of JinQuan Silver Electrolysis
Advantages of JinQuan Silver ElectrolysisAdvantages of JinQuan Silver Electrolysis:
Closed-Loop Electrolysis: Conducted throughout as a closed process, with no fumes or splashes of electrolytic solution, ensuring a clean workplace environment.
High-Density Silver Electrolysis Process: Implements a production capability that is roughly double that of other companies using electrolytic tanks of the same volume.
Electrolytic Solution Cooling System: Incorporates a system to control and minimize the evaporation loss of electrolytic solution.
Silver Electrolysis Purification Process: Ensures less frequent replacement of electrolytic solution, thereby reducing production costs.
Direct Silver Electrolysis for Anodes: Equipment processes anode directly without needing a second melting step.
Customizable Automation Solutions: Achieves automatic unloading of copper cathodes, automatic washing and drying of copper powder, pneumatic conveying after drying, and automated ingot casting, cooling, cutting, polishing, weighing, and coding, minimizing labor intensity.







